Hokkaido Study of Environment and Children’s Health
The Hokkaido study of Environment and Children’s Health is an ongoing cohort study that began in 2002. The study consists of two prospective birth cohorts: the Sapporo (Toho hospital) cohort with one obstetric hospital in Sapporo City and the Hokkaido (large-scale) cohort with 37 hospitals and clinics in the Hokkaido prefecture. Hokkaido is the northern most prefectures and the second
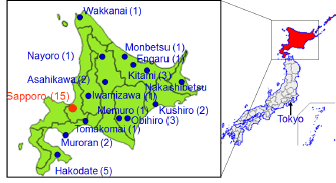
The enrollment of the Sapporo cohort (Toho hospital) was conducted from July 2002 to October 2005. The subjects were women that were enrolled at 23-35 weeks of gestation and delivered at the Toho hospital. All of the subjects were residents of Sapporo City or surrounding areas. In the Sapporo cohort, observations were focused on the association between child growth, neurodevelopment, allergy and infectious diseases, and low-level exposure to environmental chemicals during pregnancy and infancy (Figure 2).
Since February 2003, the Hokkaido (large-scale) cohort has continued to enroll women during early pregnancy (<13 weeks of gestational age) that visited one of associated hospitals or clinics in the study area for prenatal health care in the maternity unit. This cohort consists of 20,927 pregnant women as of August 2014. In total, 37 hospitals and clinics in the Hokkaido prefecture participated in the study. The Hokkaido cohort (Figure 3) was established to assess the prevalence of congenital anomalies including cleft lip and palate, congenital heart defects, hypospadias and cryptorchidism. In addition, this cohort was used to explore the possible causes of these malformations, as well as the prevalence of childhood allergies and neurodevelopmental disorders including attention deficit hyperactivity disorder (ADHD).
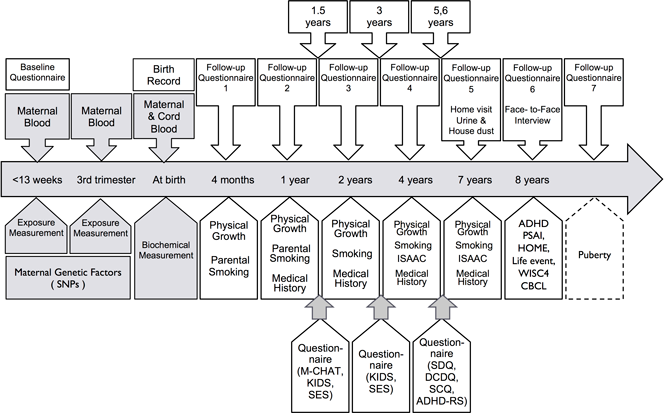
For the last ten years, we followed pregnant women and their offspring, measuring various environmental chemicals. The levels of 29 congeners of dioxins and dioxin-like PCBs (7 PCDDs, 10 PCDFs, 4 Non-ortho PCBs and 8 Mono-ortho PCBs ), 58 congeners of the other PCBs and 5 congeners of OH-PCBs in maternal blood and breast milk were measured using a high-resolution gas chromatography/high-resolution mass spectrometer (HRGC/HRMS) at the Fukuoka Institute of Health and Environmental Sciences. Eleven perfluoroalkyl acids (PFAAs) of PFCs [perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA), perfluorotridecanoic acid (PFTrDA), perfluorotetradecanoic acid (PFTeDA), perfluorohexane acid (PFHxS) and perfluorooctane sulfonate (PFOS)] were measured in maternal plasma using simultaneous analysis with liquid chromatography-tandem mass spectrometry (LC/MS/MS) and ultraperformance liquid chromatography in combination with triple quadrupole mass spectrometry (UPLC-MS/MS) at the Hoshi University, as well as Research Faculty of Agriculture, Hokkaido University. The levels of persistent organochlorine pesticides in maternal blood were analyzed by a GC/negative-ion chemical-ionization mass spectrometry (GC/NCIMS) and a GC/high-resolution mass spectrometry (GC/HRMS) at IDEA Consultants, Inc. Total mercury levels in maternal hair samples were measured by an oxygen combustion-gold amalgamation method using an atomic absorption detector at the National Institute for Minamata Disease. To determine maternal phthalate exposure levels, MEHP (a metabolite of DEHP) levels in maternal blood were analyzed by GC/MS at Nagoya University. Bisphenol A concentrations in maternal and cord blood were analyzed by isotope dilution LC-MS/MS (ID-LC-MS/MS) at IDEA Consultants, Inc. Cotinine concentrations in maternal serum were measured using an enzyme-linked immunosorbent assay (ELISA) kit to evaluate smoking exposure levels.
In the Sapporo cohort, with the purpose of assessing the neurodevelopment of the children, several behavioral examinations were conducted during each study period. The Bayley Scales of Infant Development second edition (BSID-II), the Fagan Test of Infant Intelligence (FTII), the Japanese version of the Denver Developmental Screening Tests (DDST) , the Kaufman Assessment Battery for Children (K-ABC) and the Wechsler Adult Intelligence Scale-Revised (WAIS-R), the Wechsler Intelligence Scale for Children third edition (WISC-III) and the Wisconsin Card Sorting Test (WCST-KFS version), the Evaluation of Environmental Stimulation (EES), the Japanese version of the Child Behavior Checklist (CBCL), Pre-School Activity Inventory-Japanese version (PSAI-J) were used. In addition, we also obtained the children’s medical history of atopic dermatitis, asthma, allergies, otitis media, pneumonia or bronchitis and chickenpox.
Genes that were analyzed using the SNP assay. The polymorphisms of CYP1A1 (cytochrome P450, family 1, subfamily A polypeptide 1), CYP1A2 (CYP1 subfamily A polypeptide 2), CYP1B1 (CYP1 subfamily B polypeptide 1), AHR (aryl hydrocarbon receptor), AHRR (AHR repressor), NQO1 (NAD(P)H: quinone oxidoreductase 1), CYP2E1 (CYP2 subfamily E polypeptide 1), and MTHFR (methylenetetrahydrofolate reductase) were analyzed. In addition, copy number variations (CNVs) in GSTM1 (glutathione S-transferase mu-1) and GSTT1 (glutathione S-transferase theta-1) were also evaluated.
The design of our study is a prospective cohort study intended to collect data on environmental exposures during fetal development and to control for potential confounders. The detailed measurements are adequate to detect the various effects of perinatal environmental and genetic determinants on childhood outcomes. In the Sapporo cohort study, face-to-face examinations for neurodevelopment assessment were conducted. The Hokkaido cohort had been the largest birth cohort in Japan until 2011 when the nation-wide cohort study, the Japan Environment and Children’s Study (JECS), was launched based upon our study design. Moreover, there has been an avid movement toward collaborating and integrating existing birth cohort studies across borders. The primary purpose of these birth cohort consortiums are to obtain evidence based results by using data from larger sample sizes (meta-analysis), as well as obtaining more applicable and generalizable results by integrating data beyond regions, countries and ethnicities. In Asia, the Birth Cohort Consortium of Asia (BiCCA) is now calling for participation to all existing Asian birth cohorts. [http://www.bicca.org]. Although there are many challenges associated with coordinating different cohort studies, we do believe that it is a worthy endeavor.

